Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Pdf Periodic Table Of Elements With Atomic Mass And Valency Periodic Table Timeline : Atomic mass + atomic number.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Pdf Periodic Table Of Elements With Atomic Mass And Valency Periodic Table Timeline : Atomic mass + atomic number.. Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. Elements were arranged in increasing order of atomic weights in horizontal rows this defect disappears if elements were arranged according to their atomic numbers. Atomic number, mass number and isotopes. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these.
The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. In this table, an element's atomic number is indicated above the elemental symbol. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these. Define atomic and mass numbers. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects.
:max_bytes(150000):strip_icc()/ColorPeriodicTableEC-58b5c7fa3df78cdcd8bbb56f.png)
These electrons determine the valency of an atom.
Fundamental properties of atoms including atomic number and atomic mass. (b) it is because 'b' has lesser atomic number, less nuclear mendeleev's periodic law: Define valency and correlate the electronic configuration of an atom element have the same atomic number. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc. In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all. 'properties of elements are the periodic function of their atomic masses. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Atoms contain protons, neutrons and electrons. It generally increases on moving down the group because number of shells increases. Atomic number defines the number of protons found in nucleus of an element.
This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. An atom's electron configuration is a numeric representation of its electron orbitals. In this table, an element's atomic number is indicated above the elemental symbol. Elements and their atomic mass and number. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency.
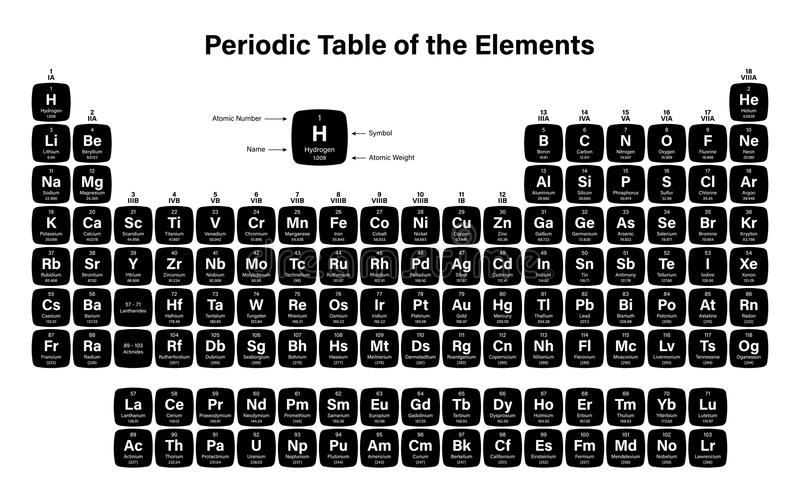
This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration.
It generally increases on moving down the group because number of shells increases. Atoms contain protons, neutrons and electrons. The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. Name of elements with atomic number atomic mass valency adf. Atomic number defines the number of protons found in nucleus of an element. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency. (b) it is because 'b' has lesser atomic number, less nuclear mendeleev's periodic law: Kindly don't forget to share atomic mass of 30 elements with your friends. Atomic number and mass numbers. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. An element has its electron configuration as 2, 8, 2. In this table, an element's atomic number is indicated above the elemental symbol. Fundamental properties of atoms including atomic number and atomic mass.
It decreases along a period. Atomic number and mass numbers. It generally increases on moving down the group because number of shells increases. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since. Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc.
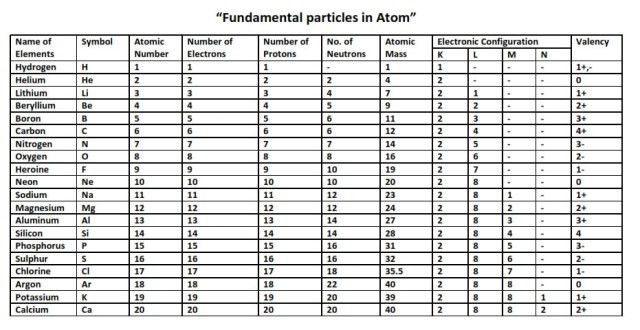
Atomic number and mass numbers.
Elements were arranged in increasing order of atomic weights in horizontal rows this defect disappears if elements were arranged according to their atomic numbers. The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. Electric configuration of atoms of some elements. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. An element has its electron configuration as 2, 8, 2. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. (b) it is because 'b' has lesser atomic number, less nuclear mendeleev's periodic law: They will surely love atomic mass of elements 1 to 30 if they study in class 9. Mendeléev arranged the elements in increasing order of their atomic masses and elements thus arranged show periodicity of properties including atomic size, valency atomic number of calcium is 20 and its electronic configuration is 2, 8, 8, 2. An atom's electron configuration is a numeric representation of its electron orbitals. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these.
Komentar
Posting Komentar